Part 2 (part 1 in the paper). would focus on NONSENSE mutations, which are rare but usually severe.
Part 1 would focus on MISSENSE mutations, which will be the vast majority of TMAU1 cases.
Speculation suggests that TMAU2 cases could also possibly be mild missense cases.
I make MISSENSE part 1 as this will be the vast majority
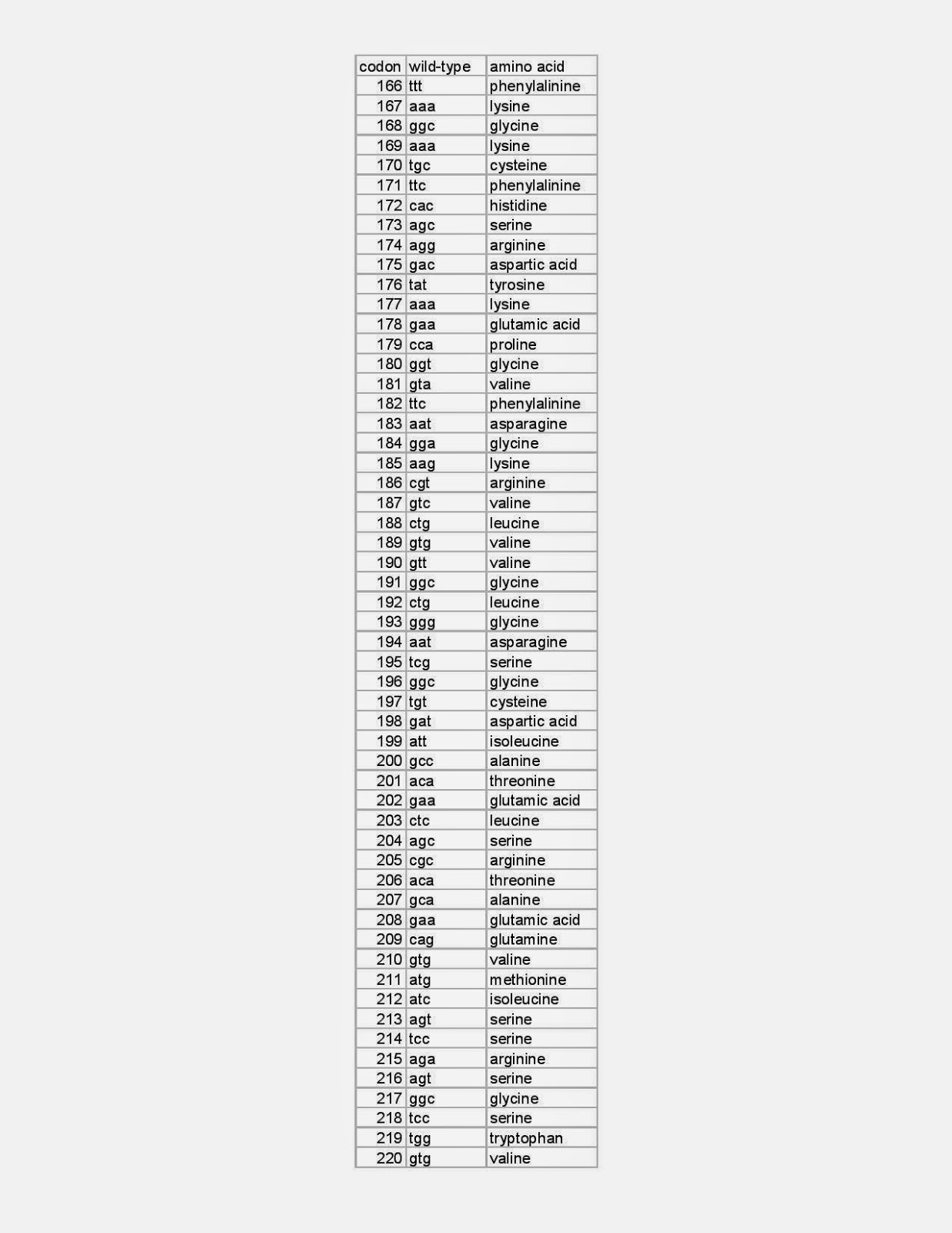
e.g. E158K E308G etc
What's needed for the study to happen :
A TRUSTED PARTY to set up a CROWDFUNDING PAGE.
Youcaring is suggested as it's free and has few clauses : Youcaring
But any crowdfund site would do.
Study cost : about $US 100,000
Why ?
Someone will be hired fulltime for 2 years.
Plus equipment etc
Study basics :
1. Using ATALUREN to see if it helps with NONSENSE mutations.
2. DRUG REPURPOSING : going through many drugs in FMO3 cells to see if any improve FMO3 function. This would help with MISSENSE mutations.
Drug Repurposing often happens in pharma-world.
Such as viagra originally being for heart disease.
Often multi-uses are found for drugs accidentally.
The study needs funding
A best option would seem to be crowdfunding.
possible scenarios :
A trusted individual or collective to take on the crowdfund.
e.g. A group of respected older women ?
or
Using the REACT Fund as a trusted proxy (if they let the consultant have the money)
RE(ACT) TMAU FUND
or
Any other ideas
Fuller outline of the potential study
Time-lines for the project (at half-yearly milestones):
Study component
|
Year 1
|
Year 2
| ||
Generation of a range of FMO3 nonsense and missense mutations and the in vitro tools for their functional analysis in a cell culture system
|
X
|
X
| ||
Evaluation of the efficacy of read-through agents and other drugs in our in vitro cell culture system, including acquisition of structural and functional evidence
|
X
|
X
| ||
2. Repurposing of existing drugs:
TMAU is caused by a functional deficiency of the FMO3 protein. Many of the missense mutations of the FMO3 gene are hypomorphs, ie there is some residual activity of the enzyme, albeit not enough to prevent the accumulation of TMA. Augmenting expression of the faulty gene through activation of the FMO3 promoter, could improve overall FMO3 enzyme expression, with significant amelioration of the disorder(19). Libraries of known therapeutic agents are commercially available that can be used in high throughput screening assays to screen for possible opportunities to “repurpose” the drug, ie apply it in a therapeutic context for which it was not originally designed.
We propose to use the cell culture models developed by us in a high throughput screening approach to identify new potential therapeutic agents that could augment expression of the FMO3 gene.
If we demonstrate potential in vitro efficacy of specific well-established therapeutic agents, we will then potentially be in a position to move to clinical trials in patients with trimethylaminuria, particularly if the therapeutic agent identified has an existing therapeutic track record in other disorders.
1. Read through of premature termination mutations: (Nonsense mutations)
Premature termination or nonsense mutations arise as a result of a single nucleotide change in a gene where the change leads to the conversion of an amino acid in the protein sequence to a premature stop codon. Such mutations often result in the protein losing most if not all of its functional capacity. It was recognised a number of years ago that aminoglycoside antibiotics can force the transcriptional machinery to read through the premature stop mutations, and allow the normal protein to be made, restoring activity of the protein(13). However, aminoglycoside antibiotics have significant side effects and are not a viable therapeutic option. More recently a new class of drugs has been developed that has the capacity to promote read through of premature termination mutations, and which appear to be totally non-toxic(14). One in particular, PTC124, has been shown to result in the production of normal dystrophin in the mdx mouse model of Duchenne muscular dystrophy(14), and has been used in clinical trials in human subjects with cystic fibrosis, with clear benefits being found(15). In addition, there are a number of other read-through agents currently being evaluated for potential in vitro and in vivo use. An inborn error of metabolism like TMAU would be an excellent candidate for this type of therapy, as an increase of enzyme activity to perhaps as little as 10% of normal should be enough to overcome the biochemical block.
Get new posts by email





 FMO3 has been taught that it cannot be induced as most other redox enzymes can (e.g. the CYPs).
FMO3 has been taught that it cannot be induced as most other redox enzymes can (e.g. the CYPs).